Immunochemical assays
Nitrocellulose-based assays and immunoprinting
Overview of method for immuno-dot assays/tissue printing
- Mark out assay sites on an appropriately-sized piece of nitrocellulose membrane (Schleicher & Schuell) by carefully ruled lines with a soft pencil. We use 5 mm x 5 mm squares. 1 μl aliquots of test compounds dissolved in water or appropriate buffer are applied to nitrocellulose and left to dry for 1 hr. It is useful to use a dilution series. Alternatively, make a tissue print of sectioned plant material or prepare a print of a displayed root system to study polysaccharide exudates.
- Incubate sheet in 3% (w/v) milk protein in phosphate-buffered saline (MP/PBS) for 1 hr to block all binding sites. It may be useful to include 0.1% (w/v) sodium azide at this step to block endogenous peroxidases.
- Incubate in primary antibody for 1.5 hr. Rat hybridoma supernatants are diluted at least 10-fold in MP/PBS.
- Wash extensively in PBS. In most cases we find that tap water can be a suitable alternative to PBS.
- Incubate in secondary antibody (e.g. anti-rat IgG(whole molecule) coupled to horseradish peroxidase (HRP), Sigma) diluted in the region of 1000-fold in MP/PBS for 1.5 hr.
- Wash extensively in PBS. See step 4.
- Determine antibody binding to the nitrocellulose by the incubation in enzyme marker substrate prepared immediately before use (e.g. HRP susbstrate: 25 ml de-ionized water, 5 ml methanol containing 10 mg/ml 4-chloro-1-naphthol, 30 μl 6% (v/v) hydrogen peroxide). prepared immediately before use) and allow to develop.
Stop reaction when dark dots have appeared at antigen sites, by washing extensively in tap water.
Microtitre plate-based assays (ELISAs)
Overview of method for antibody-capture ELISA
- Adjust a sample that is to be used as an immobilised antigen to a concentration of c. 50 μg/ml in 50 mM sodium carbonate, pH 9.6.
- Add 100 μl of antigen to appropriate wells of a microtitre plate and incubate overnight at 4oC (or 2 hr at RT). It is useful to have wells without antigen to determine signal with no antibody binding.
- Remove solutions containing antigen with washing.
- Block all binding sites on the plate with 200 μl/well 3% (w/v) bovine serum albumin in phosphate-buffered saline (BSA/PBS) for 2 hr at RT or O/N at 4oC.
- Wash plates with PBS. This is most readily done by the repeated submerging of plates in trays full of PBS, shaking and then forcibly throwing the PBS out (into a sink). This is repeated ~ten times. In our hands, tap water can often be a suitable alternative washing medium.
- Add 100 μl/well of the primary antibody at appropriate dilution in BSA/PBS for at least 1.5 hr. As a guide, rat hybridoma supernatants are diluted 10-fold followed by 5- or 10-fold dilutions.
- Wash plates fifteen times as in step 5.
- Add 100 μl/well of secondary antibody (e.g. anti-rat IgG(whole molecule) coupled to horseradish peroxidase (HRP), Sigma) diluted in the region of 1000-fold in BSA/PBS.
- Incubate for at least 1.5 hr.
- Wash plates fifteen times as in step 5.
- Determine antibody binding to the plates by the addition 150 μl/well of HRP substrate prepared immediately before use (18 ml de-ionized water, 2 ml 1 M sodium acetate buffer pH 6.0, 200 μl tetramethylbenzidene, 20 μl 6% (v/v) hydrogen peroxide).
- Colour development is stopped by the addition of 50 μl/well of 2.5 M sulphuric acid.
- Absorbances are determined at 450 nm in a microplate reader.
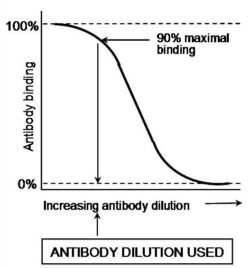
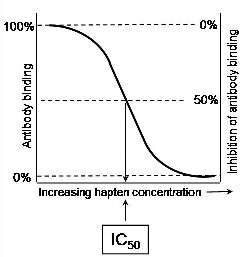
In the second type of ELISA, known as competitive-inhibition ELISA, the antigen-antibody interaction quantified is entirely in the soluble phase. Competitive-inhibition ELISA can provide a very sensitive quantification of all antigens. It is a particularly suitable technique for the assay of antibody binding to haptens or small molecules that do not stick efficiently to microtitre plates. Competitive-inhibition ELISA must be carried out in two steps as shown in the Figs A & B. First of all, the working dilution of the antibody is determined using an antibody-capture ELISA and the titration of the antibody against constant levels of immobilised antigen (see above & Fig. A). An antibody dilution that provides 90% of the maximal binding is used, in the second step, as at this dilution the antibody binding is most sensitive to inhibition. The inhibition of this level of antibody binding to the immobilised antigen by the presence of soluble antigens or haptens is determined as described below and as shown diagrammatically in Fig. B.
Overview of method for competitive-inhibition ELISA
-
Microtitre plates are coated with antigen (the immobilised antigen) and blocked with BSA/PBS.
- Determine a primary antibody dilution (x) that gives 90% of maximal binding with this antigen in antibody capture ELISA (see above & Fig. A).
- Add 50 μl/well of a serially-diluted soluble antigen or hapten in PBS to appropriate wells of a microtitre plate. As a guide, start at 2 mg/ml and prepare 10-fold dilutions.
- Add to each well containing antigen from step 3, 50 μl/well of primary antibody in BSA/PBS at a concentration of 2x. This will give a final concentration of antigen of 1 mg/ml (with 10-fold dilutions) and an antibody concentration of x. Agitate the microtitre plate gently to ensure mixing.
- Incubate for at least 1.5 hr.
- Wash plates with PBS. This is most readily done by the repeated submerging of plates in trays full of PBS, shaking and then forcibly throwing the PBS out (into a sink). This is repeated at least fifteen times. In our hands, tap water can often be a suitable alternative washing medium.
- Add 100 μl/well of secondary antibody (e.g. anti-rat IgG(whole molecule) coupled to horseradish peroxidase (HRP), Sigma) diluted in the region of 1000-fold in BSA/PBS.
- Incubate for at least 1.5 hr.
- Carry out steps 8 to 13 in antibody-capture ELISA protocol above.
Determine the concentration of antigen required to inhibit antibody binding by 50% as shown in Fig. B. This is known as the IC50. Comparing the IC50 of a range of antigens gives a good indication of extent of their recognition by a particular antibody.