Carbohydrate-binding modules (CBMs)
Carbohydrate-binding module (CBMs) are protein modules from microbial glycoside hydrolases that have the capacity to bind specifically to polysaccharides.
A CBM is defined as contiguous amino acid sequence within a carbohydrate-active enzyme with a discreet fold having carbohydrate-binding activity that is distinct from other non-catalytic sugar binding proteins such as lectins and sugar transport proteins. CBM protein modules can range in length from 40 to 150 amino acids.
Currently, over 90 different families of CBMs are recognized in the CAZy (Carbohydrate-Active enZYmes) database: CAZy - CBM (www.cazy.org)
CBMs were previously classified as cellulose-binding domains (CBDs) based on the initial discovery of modules that bound cellulose. However, modules are being found that bind polymers other than cellulose yet otherwise meet the CBM criteria which has led to the use of a more inclusive terminology. These other polymer groups include xylans, mannans and non-cellulosic glucans.
A review of the molecular basis of CBM recognition of glycans is Gilbert et al., 2013.
Our research on CBMs was carried out in collaboration with Prof. Harry Gilbert at Newcastle University. Recombinant protein engineering of CBMs to produce soluble forms with polyhistidine tags led to the development of flexible molecular probes for cell wall polymers that can be used in procedures equivalent to those used for monoclonal antibodies (McCartney et al., 2004). The differential capacities of a series of xylan-binding CBMs to recognize xylans in secondary cell walls in sections of a range of plant materials has indicated that CBMs from diverse families have distinct abilities to bind to xylans in the context of cell wall composites (McCartney et al., 2006). We have also used CBMs alongside xylan-directed monoclonal antibodies to study the recognition and degradation of xylan polymers in both primary and secondary cell walls (Hervé et al., 2009).
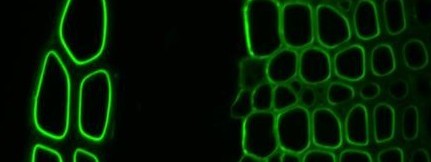
Xylan-directed CBM2b-1-2:GFP binding to TS tobacco stem
CBMs that are components of cell wall polysaccharide hydrolases are proposed to potentiate the action of the attached catalytic modules by increasing access to appropriate sites within compacted cell wall composites. The recent discovery that family 35 CBMs can bind to the product of pectate lyase action and thus direct catalytic modules to regions of cell walls where pectin degradation has occurred (Montanier et al. 2009) is a nice example of a mechanism whereby CBMs can contribute to the concerted degradation of cell walls by sets of enzymes. Using diverse fusions of catalytic modules with CBMs we have demonstrated the role of CBMs in the access to glycans and their degradation in the context of intact cell walls (Hervé et al., 2010).
CBMs that are directed to crystalline cellulose or to amorphous cellulose in vitro also show capacities to discriminate between cell walls of distinct cell types and of different species indicating high levels of variation in cellulose microstructures within cell walls (Blake et al., 2006).
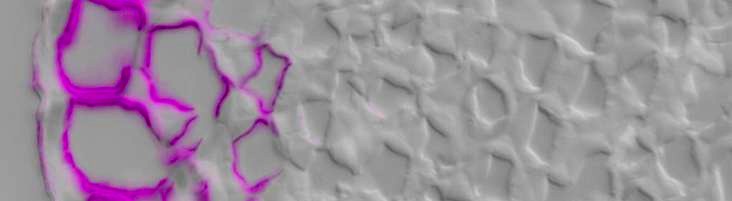
Cellulose-directed CBM9-2:FITC (purple) binding to epidermis
& collenchyma inner cell walls in TS celery petiole / DIC image
