Cell expansion & the growth of plants
The growth of all plant organs comprises the proliferation of cells in meristems, the expansion, often dramatic elongation, of cells outside meristems & the differentiation of functional cell types. The rapid increase in the size of cells proximal to shoot and root apical meristems (associated with an increase in the volume of the vacuole), is the major contributor to the growth of plants. Internal hydrostatic or turgor pressure, generated due to the presence of primary cell walls with tensile properties, is the driving force of cell expansion. Evidence indicates that the control of expansion processes resides in the modulation of primary cell walls and not through modulated turgor pressures.
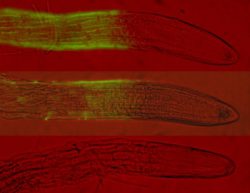
Three intact Arabidopsis seedling lateral roots immunofluorescently labelled with monoclonal antibodies JIM5 (top), LM5 (middle) and no antibody (bottom). LM5 indicates a distinct band of cell wall pectic 1,4-galactan proximal to the root tip & this marks the acceleration of cell elongation. (McCartney et al., 2003)
Most plant cells remain fixed to their neighbours as they expand but an unadhered cell or a cell at the surface of a plant organ is free to have a focused point of growth and can rapidly construct a extended tube. This mode of growth known as tip growth is observed in pollen tubes, epidermal cell root hairs and in moss protonemal cells. Mechanisms whereby contiguous adhered cells in tissues and organs elongate together remain unclear - although individual cell layers may be load-bearing and the other layers may expand upon release of constraints.
Primary cell walls are networks of cellulose microfibrils connected together by cross-linking glycans (CLGs) that are thought to have regions hydrogen bonded to microfibril surfaces. This microfibril-CLG network is embedded in matrix of complex pectic polymers. The direction of cell expansion, i.e. whether longitudinal or radial, is constrained by the net orientation of the cellulose microfibrils & cells expand at right angles to this. The extent to which a cell expands involves the modulation of the microfibril-CLG links. A range of cell wall enzymes including expansins, yieldins and hydrolases can modulate these in various ways. Recent evidence also implicates oxygen free radicals in these processes.
Primary cell walls of elongating cells are maintained at the same thickness and therefore the biosynthesis of cell wall polymers and their assembly into the integrated fibrous composite biomaterial that is the primary cell wall is a major aspect of cell expansion. Cellulose microfibrils are constructed directly at the plasma membrane and this must be integrated with CLGs and pectic polymers arriving from the Golgi apparatus & their deposition in the cell wall. There is some evidence that pectin is crucial for this assembly stage but specific contributions of the pectic polymers homogalacturonan, rhamnogalacturonan-I and rhamnogalacturonan-II to these processes are far from clear. We have identified and are studying a transient occurrence of pectic 1,4-galactan in cell walls of the Arabidopsis seedling root that marks the onset of a rapid acceleration of cell elongation in this organ (McCartney et al., 2003).
Several other factors are implicated in the regulation of cell expansion but mechanistic links are not yet clear. The arabinogalactan-proteins (AGPs) are complex proteoglycans associated with all plant cell surfaces. We are studying AGP function using AGP-binding b-glucosyl Yariv reagent that is a synthetic phenylglycoside inhibitor of AGP action that blocks the rapid phase of cell elongation in Arabidopsis seedling roots and also apical cell extension in the moss Physcomitrella patens (Lee et al., 2005).
Cartoons of pectic polymers & anti-pectin antibodies (pdf), File Download