Molecular imaging of cell walls
Principles, procedures & possible pitfalls of cell wall immunochemistry
When cell wall molecular probes are used with microscopies or in quantitative microtitre-plate- or nitrocellulose-based assays they need to be visualized. The detection of bound probes is often by means of immunochemistry procedures. Molecular probes can be coupled directly to marker molecules such as fluorophores (e.g. FITC) and then used in one step incubation procedures. This can be very effective as for example in the binding of a xylan-directed GFP:CBM2b-1-2 construct to secondary cell walls (phloem fibres left, xylem right) in a section of tobacco stem shown below. This approach requires the individual preparation of marker-coupled probes which is not straightforward for antibodies.
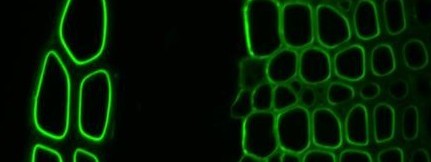
Xylan-directed CBM2b-1-2:GFP binding to TS tobacco stem
Indirect, or two stage, immunochemistry procedures are often easier, are more widely used and have the inbuilt advantage of allowing easy multi-probe comparative controls. Indirect immunochemistry procedures involve the first stage incubation of a primary probe (antibody or CBM) and, after washing away unbound probe, bound probe is detected by a secondary detection probe that is usually a further antibody. For a rat monoclonal antibody such as anti-xylan LM11 this can be anti-rat-immunoglobulin-linked to-FITC or anti-his-tag for CBM detection. This system allows the facile assessment of several probes which can often act as controls for each other as well as easy assessment of the signal from a no antibody control (that is needed to see the extent of autofluorescence of cells walls that can be a problem in some systems). The system also avoids the possible varied efficiencies of direct coupling procedures with a wide set of probes. Shown below are two primary antibodies binding to equivalent sections and detected with identical secondary antibody incubation procedures. A vast array of fluorophores and secondary detection reagents are available commercially allowing varied detection strategies. The same pot of antibody can be readily used for fluorophore or gold or enzyme detection strategies without the need to prepare specific coupled probes.
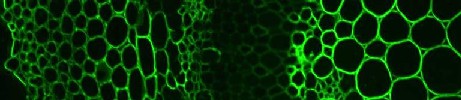
anti-arabinan LM6 / anti-rat-IgG:FITC
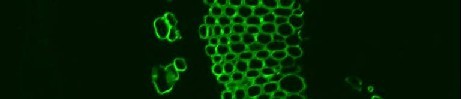
anti-xylan LM11 / anti-rat-IgG:FITC
Interpretation of epitope absence
Immunocytochemical / immunohistochemical analyses of sections of plant materials are often done with fluorescence and gold detection in conjunction with light and electron microscopies respectively. A section through a plant organ can reveal all cell wall layers from the plasma membrane to intercellular matrices of all cells. It is becoming clear however, that if a probe does not bind to a cell wall the interpretation is not as straightforward as perhaps previously thought. The lack of probe binding may indicate the presence of no antigen or low levels of an antigen. Alternatively, it may indicate that an antigen is soluble and is lost during the extensive washing steps inherent in immunolabelling procedures. This is the case with some pectins and these may be detected by tissue/organ printing techniques that pick up soluble antigens. Another possibility for the lack of a probe binding may be that the epitope is masked by proximity to or possibly covalent association with another polymer. This has recently been shown to be the case for xyloglucan in some cell walls where xyloglucan epitopes are revealed by pectic enzyme deconstruction of cell walls (Marcus et al. 2008). Work with xylan-directed CBMs indicate that polymer context within cell walls, varying between cell types and species, can also influence probe recognition (McCartney et al. 2006).
Ideally, immunohistochemical studies should consider some of these aspects and a researcher should also be wary that some polysaccharide epitopes can be present on more than one set of polymers. For example, the LM6 1,5-arabinan epitope can occur in pectic rhamnogalacturonan-I but also, in some instances, in arabinogalactan-proteins (Willats et al. 1998; Lee et al. 2005). Epitope biochemical context in an organ/cell system being studied can be determined by using the probe in complementary procedures involving biochemical or chromatographic procedures.
Molecular probes are not restricted to use with sections of plant materials and are highly effective with intact cells and organs in a wide range of systems using similar immunolabelling procedures.(See Willats et al. 2001; Willats et al. 2004; McCartney et al. 2003; Lee et al. 2005). The detection of the LM8 xylogalacturonan epitope in detaching root cap cells at the surface of an intact carrot root apex is shown below.
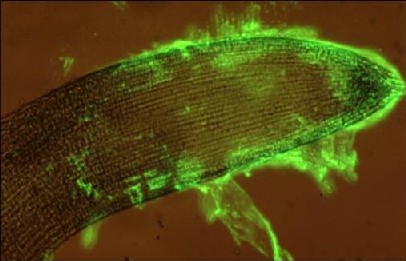
anti-xylogalacturonan LM8 / anti-rat-IgG:FITC
Autofluorescence
Some plant materials with high levels of cell wall phenolics, such as grasses, can be highly autofluorescent across all major wavelengths used for fluorophore detection. This is easily solved by the staining of sections with Toluidine Blue O after all the antibody labelling steps (Xue et al. 2013).
